The In Silico World project, coordinated by the Alma Mater Studiorum – Università di Bologna and funded by the European Union’s Horizon 2020 research and innovation programme, had its first-period Review Meeting with the European Commission officers.
The Review Meeting involved all 14 partner institutions that constitute the international consortium and further the development of 11 solutions for in silico trials, designed to test the safety and/or the efficacy of medical devices, medicinal products, and even advanced therapy medicinal products such as tissue engineering constructs for regenerative medicine. The 11 solutions target medical products to treat osteoporosis, tuberculosis, multiple sclerosis, coronary stenosis, cerebral aneurysms, mammary carcinoma, and covid-19 infection, among others.
Our consortium is also producing data collections for validation, regulatory pathways and technical standards; policy documents and information packages for patients, doctors, senior management in companies, etc.; computational strategies to make such simulations more powerful and efficient; new curricula to educate the workforce on the development and use of in silico trials technologies; and robust business models for the commercial exploitation of these technologies. All with focused attention to all legal and ethical implications of such disruptive technologies.
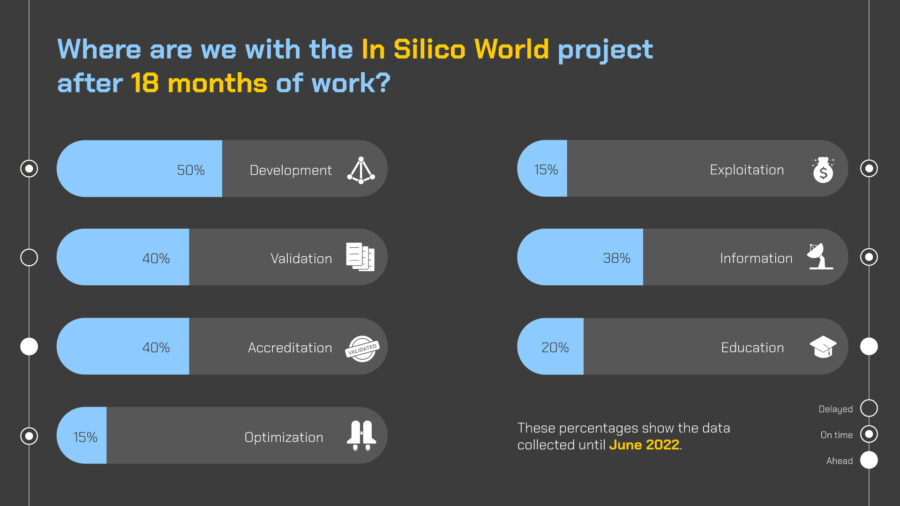
InSilicoWorld-PressRelease-Status of the project m18
Every partner presented the state of progress of its activities receiving positive feedback from the commission. We succeeded to achieve all critical objectives and milestones and the progress report provided solid evidence that the project is progressing as scheduled and the individual consortium members are working in a coordinated and efficient way towards reaching the project objectives. All this reflects a very clear understanding of the potential of in silico clinical trials and the challenges ahead, which range from the establishment of case scenarios to engaging with regulatory agencies. Also, the dissemination, exploitation, and impact activities have been achieved in line with the project objectives, targeting the healthcare professionals including a pilot survey amongst clinicians to assess their knowledge of in silico medicine as well as their expectations and fears.
For the EC Officers, the In Silico World is an ambitious, well-managed and high-impact project with high scientific and economic values. The quality of the work developed so far puts the Consortium in a strong position to advance the state of the art and make an impact in the growing field of in silico clinical trials.
“I got good reviews for my projects over the years, but never as good as this one. Amazing. – says Marco Viceconti, Professor at Alma Mater Studiorum University of Bologna and coordinator of the project – I am very proud and honoured to lead you in this beautiful adventure called In Silico World.”



CALL SC1-DTH-06-2020
The project was funded by the EC under the H2020 Call SC1- DTH – 06 -2020 (grant ID 101016503). The topic of this call is focused on accelerating the uptake of computer simulations for testing medicines and medical devices, to which the EC dedicated a budget of € 6-8 M for proposal.





